AstraZeneca COVID Vaccine Still Works, Even Though Their Marketing Tactics Don’t
Thursday, March 25th, 2021
- Clinical trial monitors for AstraZeneca’s COVID vaccine claim that data was outdated and potentially misleading.
- Yet, the data AstraZeneca released still shows that the vaccine is 76% efficacious at preventing symptomatic COVID.
- The way AstraZeneca handled the data release was a PR disaster, but the data has not materially changed. AstraZeneca’s vaccine is about 76% effective.
- The USA has sufficient supply of vaccines produced by other companies. Other countries like Canada and the UK will have to rely on AstraZeneca much more.
Pharma giant AstraZeneca has been under fire recently for controversy of their COVID vaccine. At first, concerns about the safety of the vaccine were questioned when blood clots were reported after receiving the shot. The Netherlands, Norway, Iceland and Denmark went as far as suspending use of the vaccine due to the clotting.
However, the company came out with a press release in mid-March claiming that, from a review of >17 million people vaccinated in the European Union and UK, there is no increased risk of blood clotting.
AstraZeneca’s Latest Marketing Misstep
Less than two weeks later, independent monitors of the vaccine trial put into question the validity of the data released by the company. They claimed that it was “outdated and potentially misleading”.
While AstraZeneca claims that their vaccine is 79% effective, the monitors found that, with addition of an extra month of the trial, the efficacy actually ranged from 69%-75%. The Data and Safety Monitoring Board (DSMB) feels as though the company put out data that is most favourable towards their vaccine, rather than the most recent and updated.
As a response, AstraZeneca primary analysis on early Thursday morning and the results were still consistent, but not identical, with their interim analysis. The Phase III trial of their candidate, AZD1222, in a North American study showed “76% efficacy at preventing symptomatic COVID and 100% efficacy at preventing severe disease and hospitalization”. The trial included >32,000 participants, 190 of which had symptomatic COVID. It was well tolerated with no safety concerns identified by the DSMB, and even showed 85% efficacy in those who are 65 years and older.
So What Really Changed?
The number of COVID cases included in the “Primary Analysis” was higher than the “Interim Analysis”. As a result, efficacy fell from 79% to 76%. Not a material difference.
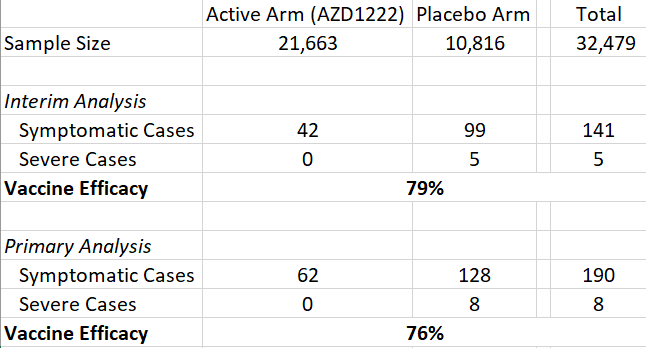
It is also important to note that efficacy in the 65+ age rose from 80% in the “Interim Analysis” to 85% in the “Primary Analysis”. Again, not materially different.
However, there are 14 additional possible or probably cases that have not been included in either analysis and may still cause the efficacy number to fluctuate a bit. This would increase the total number of COVID cases from 190 to 204. Below is how the efficacy number would change in 3 different scenarios.
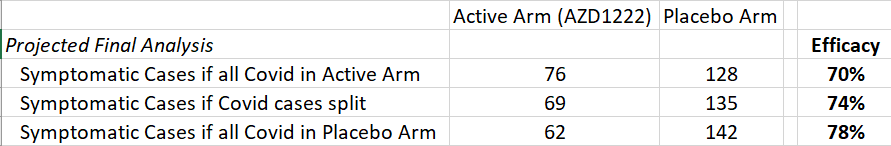
As you can see, the numbers do not change materially. The most likely scenario is that the 14 COVID cases are split, which would result in a vaccine efficacy somewhere in the mid-70% range.
What Does It All Mean for AstraZeneca’s Vaccine?
AstraZeneca plans to submit their vaccine for Emergency Use Authorization (EUA) with the FDA sometime in mid-April. Before the FDA grants AstraZeneca approval, full details of the vaccine development program will be available in the briefing documents ahead of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting. This is where an FDA committee will review and evaluate the vaccine data.
In the USA, AstraZeneca’s VRBPAC meeting will not materially change much for Americans looking to get dosed. Currently, there is sufficient supply of Pfizer, Moderna and Johnson & Johnson’s COVID vaccines available to Americans.
The same cannot be said for other countries such as Canada, the UK and EU. These countries will be more reliant on AstraZeneca’s vaccine due to the lack of available supply of the other COVID vaccines.
AstraZeneca will release the results of their analysis in late March. Vaccine efficacy is expected to come in the mid-high 70% range, with no significant safety concerns. For those willing to wait, a full data review of AstraZeneca’s vaccine will be provided by the FDA’s VRBPAC meeting in April. However, those with limited options have to remember that in times of a pandemic, any vaccine is better than nothing.