Drug Approval of Aducanumab for Alzheimer’s Gets Shut Down by AdCom, Now the FDA Must Decide
November 10th, 2020
- Biotech company, Biogen, was put under the knife regarding their drug aducanumab for the treatment of Alzheimer’s disease (AD)
- The FDA organized a meeting with an AdCom to discuss the data presented by Biogen where the AdCom voted against the drug for AD
- Biogen now waits for final decision by FDA in March 2021
Biogen’s aducanumab has the potential to be the first approved drug able to slow the progression of Alzheimer’s disease (AD). Read about the FDA’s process for controversial drugs such as aducanumab.
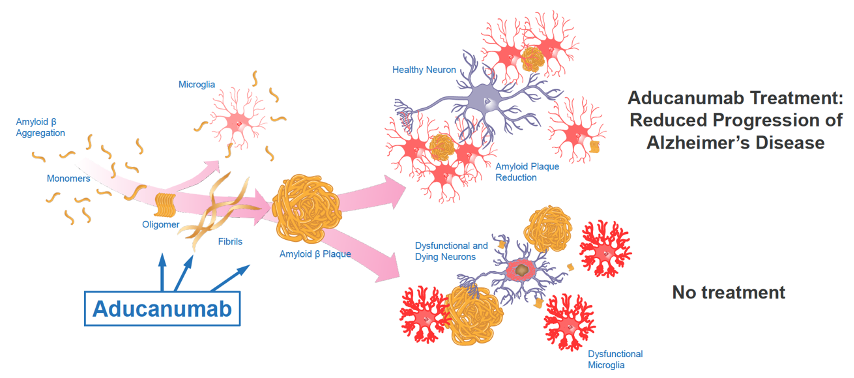
AD is a debilitating neurological disorder that progresses into mood changes, distorted thinking and is the main cause of dementia. It eventually leaves those affected with the inability to live independently, unable to perform the simplest of tasks. The idea behind the disease is that a protein called beta amyloid abnormally forms plaques around brain cells while another protein, tau, tangles itself within brain cells. AD, along with other dementia diseases, affects more than 40 million people worldwide with no cure to date.
Currently, the main focus of treatment is on maintaining the symptoms of the disease using cholinesterase inhibitor drugs such as Razadyne and Aricept. Their exact mechanisms of action towards treating AD are unclear but research suggests that they inhibit the breakdown of acetylcholine, an important chemical in the brain related to learning and memory.
Biogen Submits Application for FDA Approval with Subpar Data
Biogen’s goal was to develop aducanumab for the treatment of AD and get FDA approval for market. It is a monoclonal antibody that binds to and eliminates beta amyloid plaques in the brain. AD is one of the largest and fastest growing diseases in the US so if aducanumab gets approved, it could transform the AD space and become one of the best-selling drugs with revenues greater than $10 billion annually.
In order for a drug to get approval for market sale in the US, companies need to show safety and efficacy data from their clinical trials. They must submit an application to the FDA with data from two large and well-controlled clinical trials. Biogen submitted this information, their EMERGE and ENGAGE studies, regarding aducanumab to the FDA. The data from EMERGE showed promising results however, data from ENGAGE failed.
After the failed data had come out, Biogen re-evaluated and announced that if interpreted a certain way, ENGAGE does, in fact, show meaningful results. This is called a post-hoc analysis.
Post-hoc analysis reaches conclusions after the data has been seen. It is usually risky and rarely endorsed by the FDA. It can be useful for researchers to come up with hypotheses for future studies but not to come up with conclusions on a present study. This is why the FDA looks for endpoints defined before the commencement of the study. It ensures there is no bias or data dredging (exploiting the data to identify conclusions that can be presented as statistically significant).
Nonetheless, Biogen decided to use post-hoc analysis, move ahead and submit their drug for approval by the FDA.
What is an AdCom & Why Did Aducanumab Need One?
The FDA has advisory committees (called AdComs) which are groups of experts that consist of scientists, researchers, and patient representatives, just to name a few. They can organize a meeting at any time during the drug review process or whenever the FDA is seeking extra advice or recommendations. They review company applications and give independent advice to the FDA regarding the data. Generally, the FDA will call upon an AdCom when the case is not clear cut. In Biogen’s case, aducanumab had mixed results, but it is targeting an indication that has not seen a breakthrough in decades. The need for a treatment is dire.
It is important to note that even with an AdCom recommendation, it is the FDA who has the final say on whether the drug is approved or not. Sometimes, the FDA will go against the AdCom recommendation.
The FDA released a long 343 page document in which FDA scientists seemed to validate the aducanumab data, claiming the results being “highly persuasive” and demonstrating the “effectiveness of aducanumab” (page 57). Since the submission for aducanumab contained difficult data to interpret, with altered analyses, the FDA saw fit to round up an AdCom for further advise on the drug. The meeting was scheduled for Friday, November the 6th, two days after the positive briefing documents were released.
Tensions were high leading up to the day because if aducanumab gets approval as therapy for AD, it would become a multibillion-dollar drug. Even if associated with certain adverse events and only a proportion of those with AD were treated with aducanumab, it would still be seen as a golden ticket. The amount of people suffering and the lack of any current therapies to slow the disease progression would make aducanumab a blockbuster drug.
However, once the members of the AdCom came together, the overwhelming majority of the vote was against the efficacy of aducanumab for AD. In fact, 10/11 panel experts voted “NO” to the lone successful study being strong enough evidence of aducanumab effectiveness.
Though the FDA originally seemed in favor of aducanumab and now the AdCom is against it, it is still unclear whether it will be approved or not. Recall that the AdCom is just a recommendation and that the final decision will be left to the FDA. The FDA’s final decision date (called “PDUFA”) has been scheduled for March 7, 2021.
History Shows That Not All Hope Is Lost Post Negative AdCom
A similar situation was seen in 2016 with drug development company, Sarepta. Their drug, eteplirsen was developed for the treatment of Duchenne muscular dystrophy (DMD), a fatal muscle degenerative disease. Similar to AD, there was an urgent need for therapy against the disease. An AdCom meeting was organized and the panelists voted against eteplirsen for the treatment of DMD. The FDA made the final decision on September 19th, 2016 to approve eteplirsen as the first drug able to treat DMD. Exondys 51, the commercial name for eteplirsen, is on pace to do $450M in sales for 2020.
As you can see, the decision to approve a drug for market sale is not easy. There are years of research and contemplating involved. Although an AdCom is very beneficial as they provide expert advice on the data, ultimately, the final decision is left up to the FDA. As seen in the DMD example, the AdCom voted against eteplirsen but in the end, the FDA decided in favour of its approval. The question now is how the data given by Biogen and the recommendation provided by the AdCom will alter the decision of the FDA towards the approval or disproval of aducanumab. Judgment day is scheduled for March 2021.