New Weight Loss Paradigm: Diabetic Drugs Showing Promise As Treatments
Monday February 22nd, 2021
- An anti-diabetic drug, semaglutide, used for treatment of type 2 diabetes has shown to help reduce 15% of body weight over a 68-week period
- In a Phase 3 study, semaglutide demonstrated superior efficacy and convenience with once weekly injection. However, the drug will likely be expensive as the lower approved dose to treat diabetes costs $1,000/month.
- The drug is under FDA review and a verdict is expected by mid-2021
- Another anti-diabetic drug, tirzepatide, has also reduced weight by 13.9% in clinical trials
An anti-diabetic drug used for treatment of type 2 diabetes has shown to help reduce 15% of body weight over a 68-week period (or 1.3 years). The drug is Ozempic (semaglutide) and sold by large pharma company Novo Nordisk. In December 2017, Ozempic was approved for type 2 diabetes as a 1mg weekly injection. While studying Ozempic for type 2 diabetes, weight loss was observed in obese patients. This prompted studying the drug as a weight loss treatment. It is now under FDA review to be used as an anti-obesity drug at a 2.5mg, once weekly dose.
The Weight Loss Study
The Phase 3 study, called STEP1, enrolled 1961 adults with a body mass index (BMI) of 30 or more. For context, a BMI between 18.5-25 is considered “normal weight” (see image below). 2/3rd of the patients received semaglutide injections once weekly and the other 1/3rd received a placebo.
In addition to the drug, participants received individual counseling sessions to help them adhere to a reduced calorie diet (500-kcal deficit per day) and increased physical activity (150 minutes per week).

The primary endpoints of this study were:
- to observe the percent change in body weight
- achievement of 5% or more reduction in body weight after 68 weeks
What did the data show?
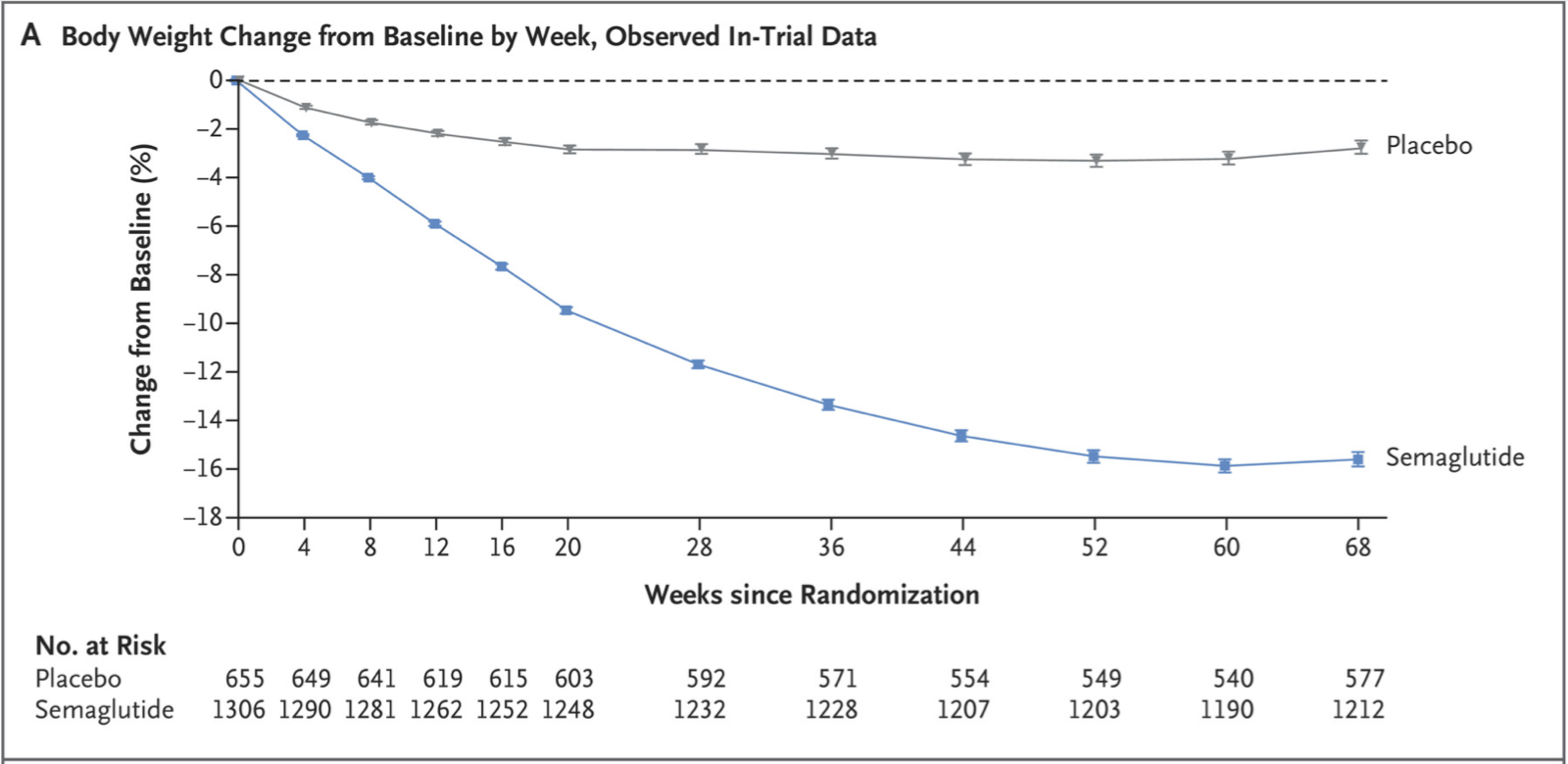
Both the primary endpoints were met. Those who got the drug lost 14.9% of their body weight, on average, compared with 2.4% among those receiving the placebo. This is a 12.4% body weight difference over 68 weeks (see below).
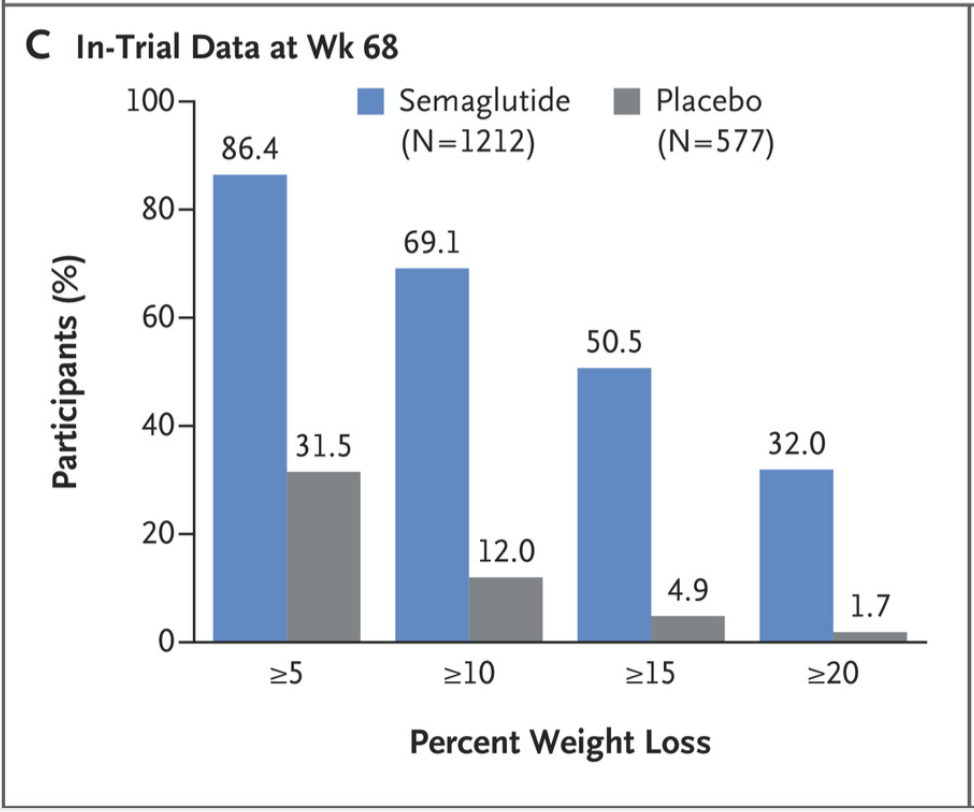
Also, compared to those who were in the placebo group, those who received 2.4 mg of semaglutide were more likely to lose 5% or more of body weight at 68 weeks. 1047 participants (86.4%) were able to achieve this threshold. The image below also shows the percentage of people that were able to lose 10%, 15% and 20% or more of body weight at the end of the trial compared to baseline.
The drug was also effective at reducing waist circumference (-13.54 cm vs. -4.13cm in placebo), BMI (-5.54 vs. -0.92) and blood pressure. Symptoms of diabetes and pre-diabetes improved in many patients.
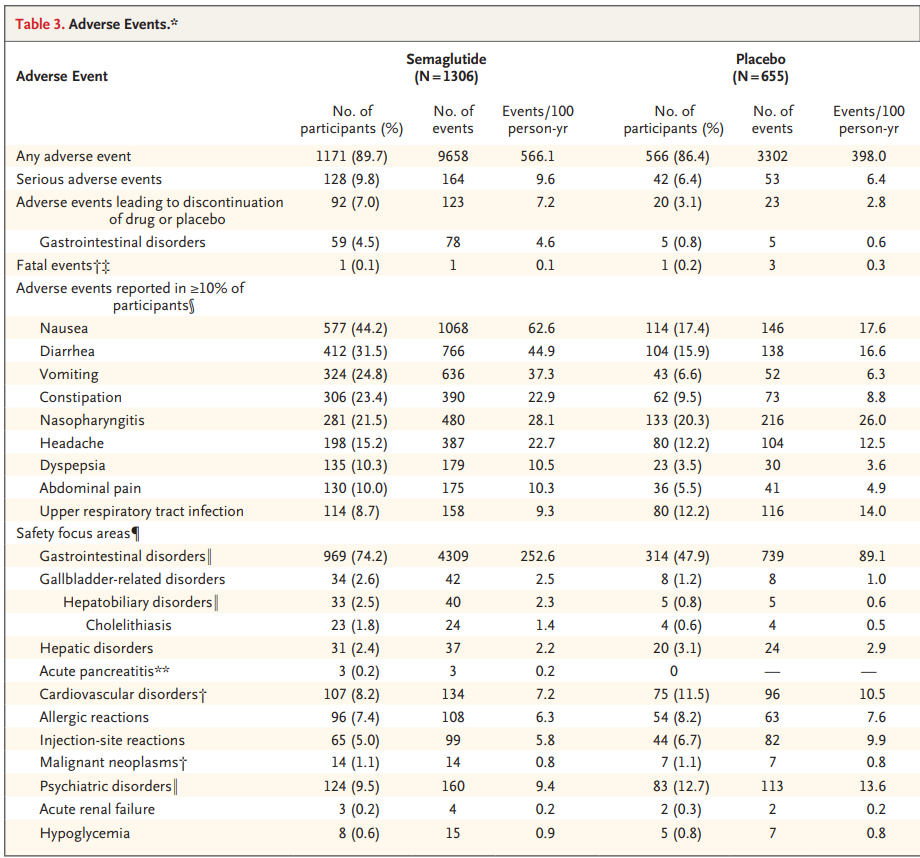
Side effects of semaglutide were relatively low as the drug showed similar adverse events to placebo. 89.7% of participants taking the drug reported an adverse event, compared to 86.4% on placebo. The most common events were nausea in 44% of patients, diarrhea in 31.5% and vomiting in 24.8%.
How Does Semaglutide Compare to Other Weight Loss Interventions
Those who are overweight or obese are recommended to lose 10-15%, or more, of their weight. This was achieved in the semaglutide group. About 70% of participants lost 10% and ~50% lost 15%. Importantly, about 30% of participants in the experimental group, were able to lose 20% of their baseline weight. This is very significant as that much weight loss is usually seen in patients 1-3 years after bariatric surgery (surgeries on the digestive system to help with weight loss).
Phentermine, another weight loss drug, demonstrated a 7.5% drop in body weight, on average, and can be taken only for a short time. After it is stopped, the weight is regained. This is also expected with semaglutide. Patients would have to take it for a lifetime to prevent the weight loss from coming back.
Another bonus of semaglutide over current approved drug interventions is that the once weekly injection of semaglutide may increase compliance compared to other approved drugs that need to be taken several times a day.
To really understand the potential superiority of semaglutide against other treatments, head-to-head trials will need to be run. The study discussed above had a few demographic limitations as well, which included only 26% males and 75% white. These demographics are not reflective of the obese population. In the US, for example, obesity is equally prevalent in both males and females and actually more prevalent in non-white adults. According to the CDC, Non-Hispanic black adults had the highest prevalence of obesity (38.4%), followed by Hispanic adults (32.6%) and non-Hispanic white adults (28.6%).
Bottom Line: Semaglutide has shown superior efficacy and convenience with once weekly injection, compared to other weight loss treatment options. However, the drug will likely be expensive as the lower approved dose to treat diabetes costs $1,000/month.
Semaglutide is a synthetic version of a naturally occurring hormone that increases insulin production and lowers blood sugars. This slows down digestion in the stomach and lowers appetite. Because the drug is relatively new, a high-dose regimen has not been studied long enough to know if it has serious long-term consequences.
Other Diabetic Drug Showing Similar Weight Loss Results
Tirzepatide, by pharma company Eli Lilly, is also being studied for use in adults with type 2 diabetes. Their clinical trials, SURPASS-3 and SURPASS-5, looked at the use of 5 mg, 10 mg, 15 mg of tirzepatide or insulin glargine (placebo) for glucose management. In SURPASS-3, the highest dose of tirzepatide (15 mg) reduced weight by 13.9% (12.9 kg) and A1C (blood glucose test) by 2.37%. In SURPASS-5, weight was reduced by 11.6% (10.9 kg) and A1C by 2.59%.
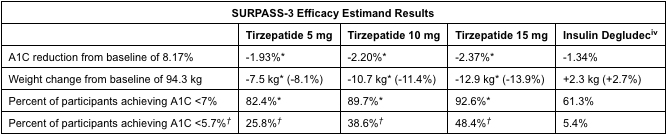
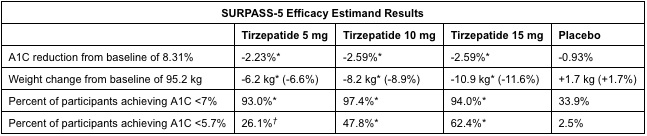
Tirzepatide, like semaglutide, is a once weekly injection. It is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. Studies have shown that GIP decreases food intake and increases energy consumption which, in combination with a GLP-1 agonist, lead to significant effects on weight and glucose. The side effects reported were mostly gastrointestinal-related and mild to moderately severe.
Both tirzepatide and semaglutide are currently in ongoing clinical trials. Tirzepatide is currently in Phase 3 for management of adults with type 2 diabetes and weight reduction. The Phase 3 SURPASS program began in 2018 and has enrolled over 13,000 participants. Results are expected in 2021.
In December 2020, the makers of semaglutide submitted an application to have it reviewed for FDA approval as a weight-loss drug. The company expects a verdict from the FDA as soon as mid-2021 and a decision in Europe by early 2022.
Both drugs seem like prospective alternatives for those who require weight management and have type 2 diabetes. Results from clinical trials show that they both significantly reduce body weight and glucose blood levels with mild side effect profiles. The next step is to hopefully get these drugs to market to provide a safe, convenient and affordable alternative to those who are overweight or obese.
A new weight-loss treatment paradigm can be upon us.