NK Cells: Oncology’s Next Big Wave
October 18th, 2020
Key Points:
- Although two CAR-T therapies have received FDA approval, shortcomings limit their usability in a real-life setting
- Allogeneic “off-the-shelf” CAR-T approaches also face two main hurdles: GvHD and graft rejection
- NK therapies, including engagers and CAR-NK, have seen a recent spike in interest due to their superior safety potential and broad manufacturing capabilities
- Next-gen cell therapy approaches are discussed, include allogeneic CAR-T & CAR-NK, NK cell engagers and the different NK cell sources
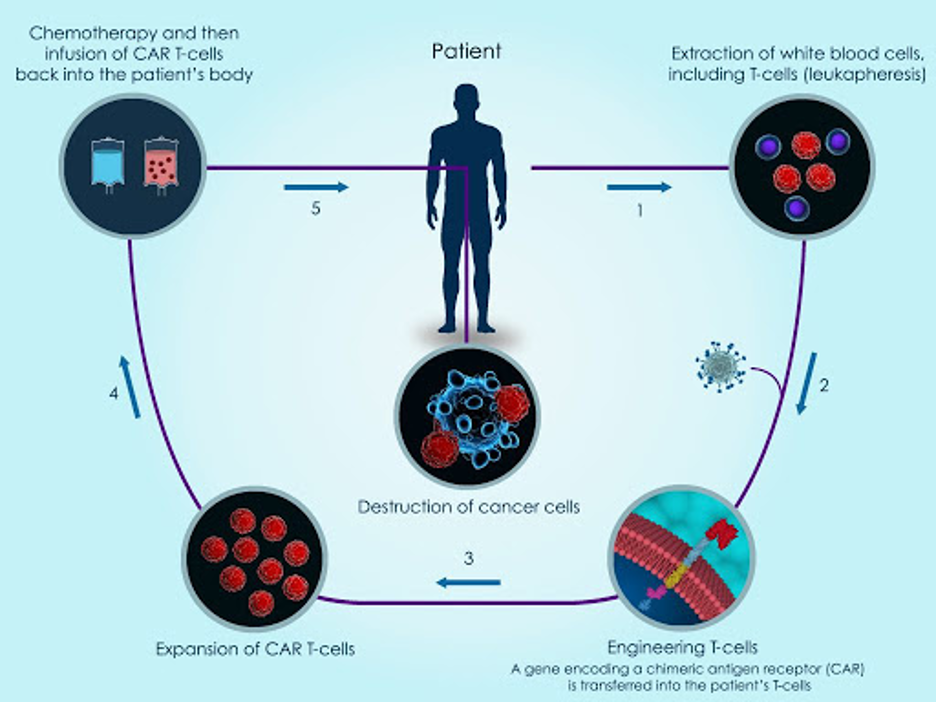
For several years, chimeric antigen receptor T-cell (CAR-T) therapy has been the topic of discussion as the next-gen treatment for oncology. CAR-T works by using T cells from the patient’s own blood, referred to as “autologous cells”. The T cells are collected, genetically modified and multiplied, and then re-injected back into the patient (see image below).
The idea behind CAR-T cells is that a synthetic receptor is attached to the T cells and when this receptor comes into contact with the antigen of an infected cell, intracellular domains activate the cytotoxicity of the T cell. In simple terms, T cells are genetically modified and enhanced to better detect cancer cells when they are reinjected back into the body.
CAR-T Shortcomings Limit Usability in Real Life Setting
Two CAR-T cell therapies have been approved by the FDA, both showing promising results. However, they come with their own set of challenges. Most people suffering with cancer, who could benefit from the therapy, are not receiving it. This is because it involves a lengthy manufacturing process with a high cost profile and could have potentially severe side effects.
Allogeneic Approach Has Answers to Existing CAR-T Limitations, But Other Issues Arise
To make CAR-T more accessible to those in need, researchers are using T cells extracted from a third-party donor (called “allogeneic”). This off-the-shelf concept is similar to the autologous method discussed above but allows for the cells to be easily accessible in the hospital setting. This way, large proportions of pre-engineered cells can be preserved for use when needed and one donor’s T cells can be administered to several patients, thus lowering the manufacturing costs and time.
There are two main hurdles with the off-the-shelf approach:
- Graft-versus-host disease (GvHD) – Occurs when the donor T cells view the patient’s cells as foreign and attack them, causing serious or fatal side effects.
- Graft rejection – Graft rejection is the opposite process. The patient’s own immune system detects the donor T cells as foreign and rapidly eliminates them, thereby limiting their anti-tumor activity.
Modifications Helping with Allogeneic CAR-T Hurdles
Cellectis and Allogene are two companies making off-the-shelf CAR-T cells genetically engineered to avoid GvHD and graft rejection. Using Cellectis’ gene editing technology, Allogene makes two modifications:
- Eliminate functional T cell receptors (TCRs) that prevent the CAR-T cell from recognizing a patient’s normal tissue cells as foreign. Without these TCRs, the CAR-T cells cannot bind to healthy cells found in the patient. This is meant to improve safety and limit the risk of GvHD.
- Knocks out the CD52 surface protein on the CAR-T cells. In addition, Allogene uses their own anti-CD52 monoclonal antibody to suppress the host immune system from recognizing CD52 on the surface of foreign CAR-T cells. Without CD52 on CAR-T & use of anti-CD52 antibody, Allogene believes that the CAR-T & patient immune system will not be attacking each other. Instead, both will work together to attack the tumor cells.
See a visual representation of the two modifications Allogene has made to control GvHD & graft rejection (below). So far in early Phase 1 studies, Allogene has not reported any cases of GvHD or graft rejection in 22 patients.

The NK Cell Approach to Treating Cancer
The above-mentioned CAR-T limitations have sparked a recent interest in NK cells as an alternate treatment approach. Early studies were focused on infusing non-targeted endogenous NK cells back into patients (non-targeted NK cells do not have any antibodies or modifications). This resulted in a lack of tumor recognition by autologous NK cells. Therefore, it was determined that a combination of NK cells + target therapies was needed.
Side Note: What differentiates NK cells from their lymphocytic counterpart T cells is their ability to kill cancer cells without sensitization. Yet, when primed with cytokines, the cytotoxic function of NK cells is increased along with their longevity in vivo.
To get a better understanding of NK cells and their basic biology, read LifeScite’s NK Cells for Dummies: Understanding the Next Cancer Paradigm article.
Combinations With NK-Cell Engagers
To improve NK cell antitumor activity, pharma companies are combining antibody targeting agents with NK cells. Affimed recently entered Phase 1 trials that will test a combination of NK cell engager AFM13 with MD Anderson’s off-the-shelf NK cells.
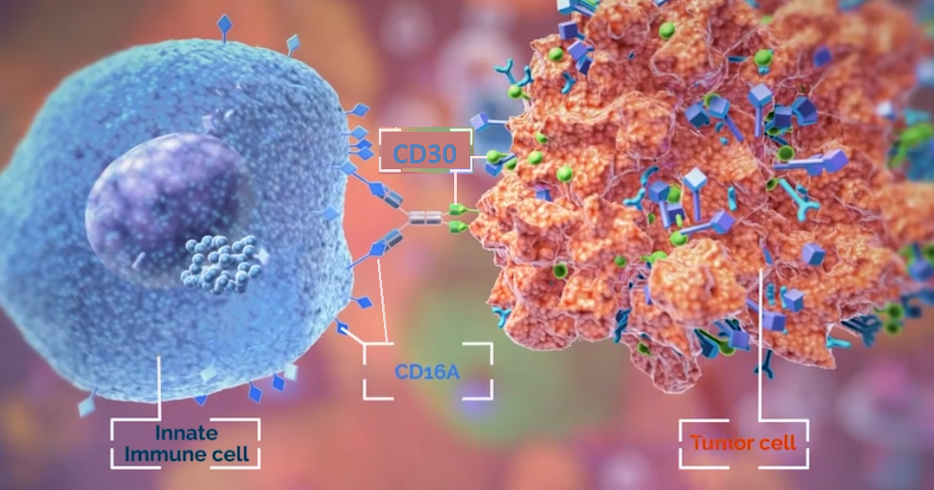
AFM13 is an antibody which binds to CD16A receptor found on NK cells and other innate immune cells like mast cells, macrophages and monocytes. The other end of the antibody will target the CD30 antigen expressed on lymphoma tumor cells (see image below). The hope is that NK cells equipped with better targeting agents will lead to added antitumor activity and therefore superior efficacy.
Similarly, Innate Pharma’s monoclonal antibody monalizumab blocks the checkpoint NKG2A receptor found on NK & certain T cells. By blocking this inhibitory receptor, Innate believes that the cancer fighting cells (NK cells and T cells) will be able to better detect, and bind, the HLA-E receptor found on tumorous cells. The company is advancing their innate checkpoint blocker into a Phase 3 study in combination with cetuximab.
CAR-NK Do Not Share CAR-T Limitations
Similar to CAR-T, CAR-NK cells are genetically equipped with receptors on their surface which seek signs of danger or stress that might indicate a tumor, or a cell infected with a virus. When they find such a cell, NK cells can attack it directly and call T cells to the site to help.
Data thus far shows that CAR-NK cells contain several benefits over their CAR-T cell counterparts:
- Superior Safety Profile: CAR-NK cells have a low risk of cytokine release syndrome compared to CAR-T cells because they secrete restricted levels of pro-inflammatory cytokines IFN-γ, IL-1 and IL-6.
- Broad Production Potential: Low likelihood of triggering GvHD upon allogeneic infusion means that CAR-NK cells may be prepared as an “off-the-shelf” product and are not restricted to autologous cells.
- CAR-NK cells can work in CAR-dependent and CAR-independent manners. (Recall that CAR stands for chimeric antigen receptor.)
- CAR-dependent: Cancer cells are smart and can make themselves “invisible” to immune cells. The CAR added onto NK cells now gives them the ability to recognize and kill a target on cancer cells that were once “invisible”.
- CAR-independent: CAR-NK cells also contain native receptors and retain their intrinsic ability to recognize and kill cancer cells independent of CARs. This is because they do not rely on antigen-specific binding to be turned on but rather, can induce a positive or negative signal through binding with ligands on target cells.
NK Cell Sources: Not All NKs Are Equal
NK cells demonstrate promising potential to be prepared as an off-the-shelf product. This, however, requires a donor source where ample, functional NK cells may be harvested. To date, NK cell isolates have most often been extracted from the donor’s peripheral blood (PB-NK cells) or umbilical cord blood (UCB-NK cells).
Obtaining an abundant number of functionally active NK cells from these sources has proven to be difficult. Other and more accessible cell sources include NK-92 cells and stem cell-derived NK cells.
Below is be a breakdown of each of the cell sources.
Peripheral blood (PB-NK cells)
These NK cells are collected from the blood of screened healthy donors. The NK cells (and specific types of T cells) are isolated from the collected blood sample, expanded, and genetically modified with editing technologies like CRISPR to improve persistence and overcome resistance.
Editas Medicine in Cambridge, Massachusetts, is using CRISPR to remove a receptor on NK cells that bind TGF-β — a signaling molecule produced by some tumors that can shut down immune cells. The hope is that removing the receptor will make the CAR-NK cells more potent tumor-killing machines.
Umbilical Cord blood NK Cells (UCB-NK cells)
NK cells isolated from umbilical cord blood have shown to possess reduced cytotoxicity when compared to peripheral blood derived NK cells. However, the use of umbilical cord blood as a source of NK is also limited in the number of NK cells that can be obtained, when compared to peripheral blood as a source.
A study out of MD Anderson (Rezvani, Liu et al. 2018) collected UCB-NK cells and engineered them with CARs that allowed the innate cells to target the CD19 protein found on select tumor cells. The modified NK cells were also made to express IL-15, a cytokine that activates NK cells, as well as inducible caspase-9 (iC9) as a safety switch. Data (found here) showed early efficacy in several lymphoma cancers and a clean safety profile. This caught the attention of big pharma company Takeda, which licensed the program in late 2019. Takeda estimates that the program can enter pivotal trials in specific refractory lymphoma indications as early as 2021 and have a BLA filing by 2023.
NK Cell Lines
A specific strand of NK cells called NK-92 are sourced from cancer patients. These withdrawn cells are irradiated so that the cancerous cells are wiped out, and only the functional NK-92 cells remain. After irradiation, NK cell lines are expanded indefinitely, and can be genetically modified to recognize specific antigens found on tumor cells. Various clinical trials with CAR-NK cells use NK92 cell line because of their ability to be expanded indefinitely in vitro and are able to undergo repeated freeze/thaw cycles needed during manufacturing process. This gives NK cell lines ideal features as off-the-shelf products.
However, because NK-92 cells are sourced from a tumor cell line they have several drawbacks including (1) potential tumorigenicity risk (ability to form into tumorous cells) (2) lack of CD16 and NKp44 expression (non-targeted to attack tumors) and (3) loss of in vivo expansion potential due to the irradiation process.
Nantkwest is developing several modified NK-92 cell lines to target cancer. Their t-haNK platform incorporates receptors (CD16, CD19, NKG2D etc.) as well as CAR to target cancer cells and enhance cell killing capabilities.
Stem cell-derived NK cells
Think of human induced pluripotent stem cell (iPSC) as a blank slate. These stem cells can be modified to become 200+ cell types, including NK cells. Stem cell-derived NK cells have the ability to produce unlimited cells for use in the clinic. Their ability to be genetically engineered and unlimited ability to self-renew gives them the potential to generate an infinite number of cells for off-the-shelf CAR-NK cells.
Fate Therapeutics has a pipeline of genetically modified iPSC derived NK cells, that include CD16 to augment antibody therapy, IL-15 to enhance NK cell function, CAR to make NK cells more targeted and CD38 knockout to improve safety. Fate’s iPSC-derived NK cells have shown to be safe and well tolerated in Phase 1 data. Fate signed a collaboration with Johnson & Johnson’s pharmaceutical arm Janssen to build novel off-the-shelf iPSC-derived CAR-NK and CAR-T candidates.

Current State of NK: Early, But Promising
It is clear that NK cells as therapy prove to be a great option for use in the clinic. They provide effective results while overcoming the GvHD associated with their CAR-T counterparts. However, their safety and efficacy has only been shown towards a handful of cancers. The challenge will be seen when solid tumors are targeted. NK cells are still a relatively new concept in the oncology field and thus, should continue to be studied in order to confirm their long-term effects. With the recent big pharma interest, it seems likely that the resources into NK cells will continue pouring in.