Omicron Variant Prolongs Temporary Pause on FDA Inspections
Friday January 21st, 2022
- Latest COVID variant, Omicron, is spreading so rapidly that the FDA had to put a pause on routine inspections since March 2020.
- Inspections are an essential part of product development as they ensure safe, effective and quality products make it to the market.
- FDA plans to extend the pause into February 2022 which will have a large impact on the availability of products for American and global consumers.
It should come as no surprise that the new COVID variant, Omicron, is sweeping the globe. The CDC just released data stating that over 95% of COVID cases in the US are caused by the Omicron variant. Though updated data is released on a daily basis, what we do know about that variant is that it spreads “more easily than the original SARS-CoV-2 virus”, according to the CDC.
The spread of Omicron has become so serious that the FDA declared they will be extending the pause on facility inspections. Originally announced back in March 2020, the FDA planned to temporarily postpone foreign surveillance inspections until April. Only those considered “mission-critical” would be considered. The decision was made to ensure the safety of their employees who would have to travel to conduct the inspections, as advised by the CDC. The FDA claimed they were confident in their ability to continue operations using alternate methods.
Postponed FDA Inspections Impact Millions of People & Trillions of Dollars Globally
It is important to emphasize the role that inspections play on public health. Each country has their own governing regulatory agency that oversees thousands of products. In the US, the FDA ensures that safe and effective products are developed for human use, including but not limited to drugs, medical devices, and biologics. The FDA regulates >$2.7 trillion in food, tobacco, and medical products. Put differently, FDA-regulated products make up 20% of consumer spending in the US. However, the FDA is not limited to products developed solely for the US. They are also heavily involved in the approval process globally.
The FDA’s Surveillance Inspections are paramount to the safe and effective production of pharmaceuticals, vaccines, medical devices and more. Postponed inspections cause supply chain disruptions, empty shelves and pricing increases at the supermarket, hospital, or pharmacy.
FDA Inspections During COVID
The FDA released a report in May 2021, “Resiliency Roadmap for FDA Inspectional Oversight”. This document outlines the FDA’s involvement in surveillance inspections along with their plan to resume them as quickly and safely as possible.
Manufacturers of FDA-regulated products are responsible for ensuring the effectiveness and more importantly, the safety, of their products. They must follow Current Good Manufacturing Practices (CGMPs) outlined by the FDA. Periodic inspections are conducted to ensure that manufacturers are adhering to these regulatory requirements. Passing an inspection is paramount to producing a product fit for FDA approval.
If any discrepancies are found, the FDA enforces corrections that must be made, and manufacturers must comply to ensure the continuation of their production. If requirements are not followed, the FDA can take corrective action, or worse, halt the manufacturing of the product completely.
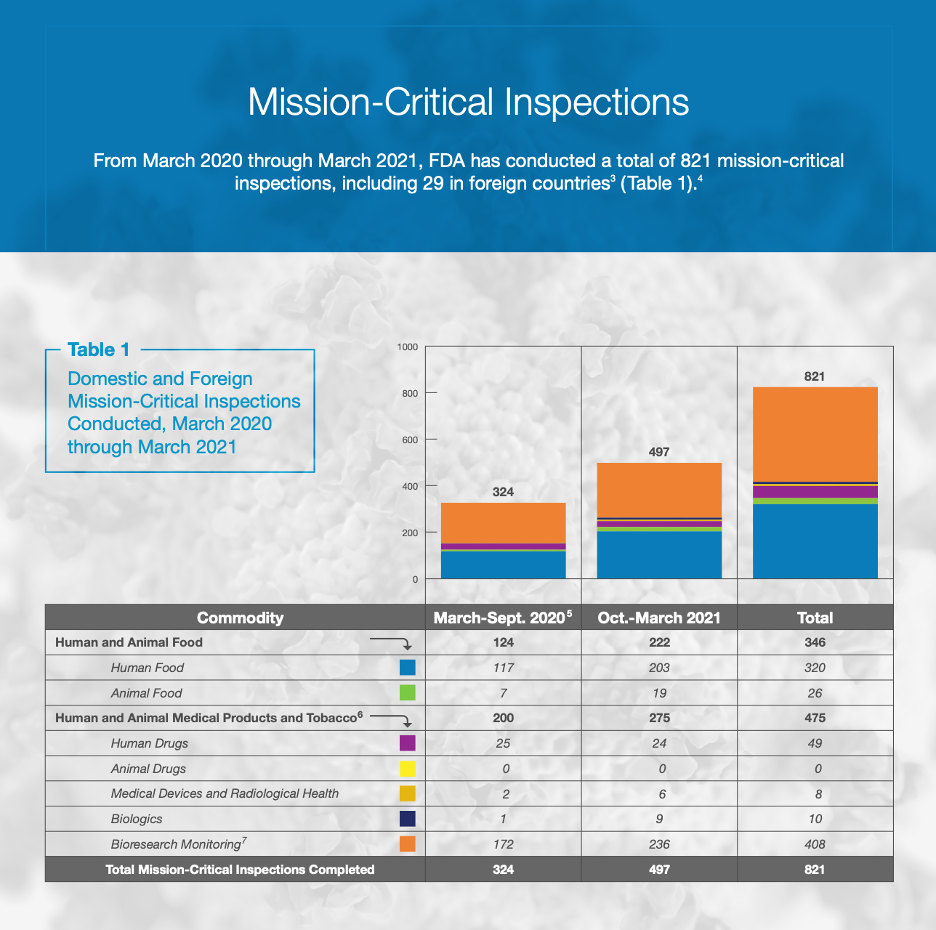
The roadmap published by the FDA includes charts outlining their progress from March 2020 to March 2021, when the changes to regular inspections were made. During that year, 821 mission-critical inspections were conducted by the FDA, 29 of which were in foreign countries.
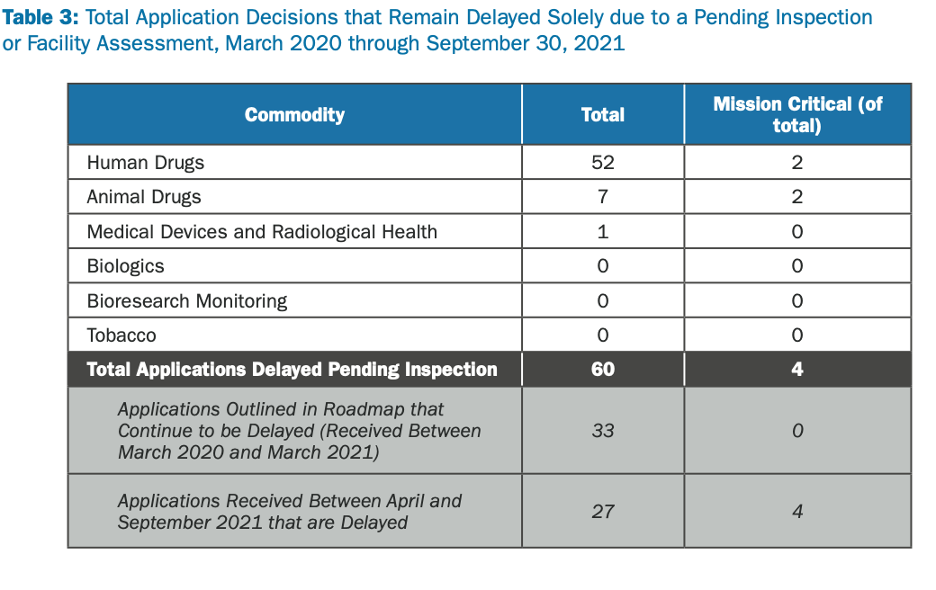
The FDA released an update in November 2021 stating that of all the applications they have received since March 2020, 60 of them are delayed or on hold because of the halt on facility inspections. Of these 60, 4 of them have been identified as mission-critical and 90% require foreign inspections. The FDA plans to resume these foreign inspections once traveling has been deemed safe from the government.
The latest update from the FDA, as of a few days ago, states that the temporary pause will continue into the beginning of February. They plan to continue foreign and domestic mission-critical inspections including remote surveillance.
The importance of periodic and continual inspections is critical for the manufacturing of safe, effective, and quality products. However, with the increased incidence of COVID variant infections, the FDA has no choice but to limit the number of in-person inspections and transition to mission-critical and remote alternatives. The FDA regulates billions of dollars’ worth in drugs, medical devices and more thus, only time will tell how these substitutions will impact the production, approval, and marketing of products for use by not only American, but global consumers as well.