Trillium’s CD47 Monotherapy Data Draws Attention of Pfizer and Wall Street
September 17th, 2020
Last week, Trillium reported topline data from their latest cohort for TTI-622, the CD47 targeting candidate. The monotherapy data triggered a series of events that followed, which included a $25M investment from Pfizer and a $150M financing from healthcare focused Wall Street funds.
Below is why Trillium’s approach is garnering attention from big pharma and Wall Street.
CD47 Approach Is Interesting Due to Broad Tumor Applicability
A large number of hematologic and solid tumors express high levels of CD47, providing a “do not eat me” signal by binding to signal regulatory protein alpha (SIRPα). This “do not eat me signal” prevents innate immune cells (first line of immune defense; NK-cells, macrophages, to name a few) from attacking tumor cells. Think of CD47 as an evasive tool that tumor cells use to stay off the radar of the immune system.
It is believed that a CD47-blocking antibody will enhance the immune system’s ability to detect tumors that are currently evading the immune system. The rapidly evolving CD47 space has seen an increase in interest recently with big money and big pharma allocating research dollars.
In addition to blocking CD47 through the SIRPα domain, companies are also adding “eat me” signals to enhance the anti-tumor activity. Companies are using different types of antibody isotypes, mainly IgG1 and IgG4, to trigger anti-tumor activity.
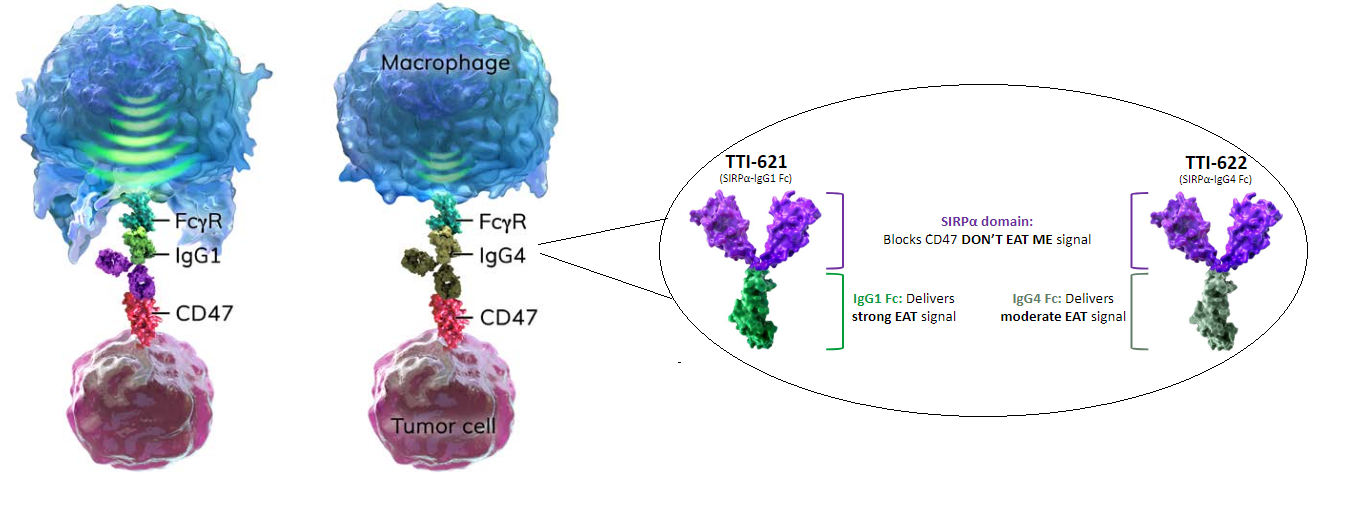
Below is a visual representation of a macrophage, representing the immune system defense mechanism, and the CD47 blocking agents that activate the immune system to detect tumor cell.
- FCγR: FC receptor found on surface of innate cells
- IgG1 and IgG4: antibody isotypes that deliver the “eat me” signals
- CD47: “do not eat me” signal found on surface of tumor cell
Gilead’s $4.9 Billion Acquisition of FourtySeven Validated CD47 Space
In early 2020, Gilead struck a deal to buy development stage company FortySeven for $4.9 billion. The takeover gave Gilead control of Phase 1 anti-CD47 antibody magrolimab. FourtySeven had reported 15/39 (38%) overall response rate (ORR) in Diffuse large B-cell lymphoma (DLBCL) patients that had received >3 lines of treatment. Importantly, the response rate was for a combination of FourtySeven’s magrolimab + rituximab. This acquisition kickstarted the interest in CD47 space.
Trillium’s Data Has Best in Class Potential
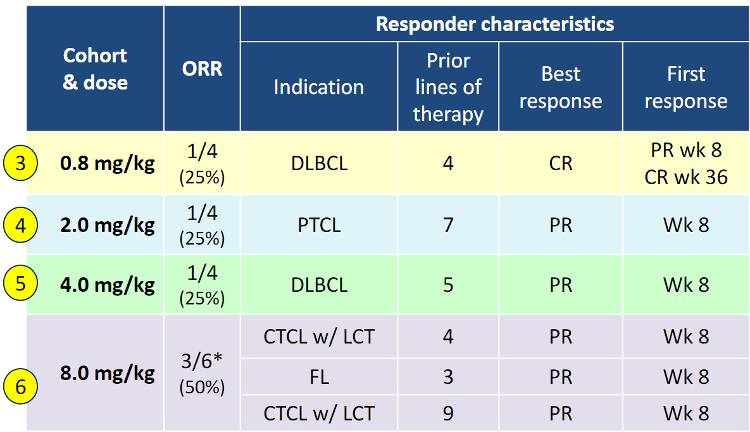
The data that piqued big pharma and healthcare fund interest came from Trillium’s latest cohort reported last week. Cohort 6 (8mg/kg) in relapsed/refractory lymphoma showed 3/6 patients (50%) experienced a partial response (PR). This data was superior to lower dosed Cohorts 3-5 (see below) which had response rates of 25%.
This is an important event for Trillium because these responses are in heavily pre-treated patients (with at least 3 prior lines of therapy) and demonstrate monotherapy activity across a broad range of lymphoma indications. In all Cohorts tested to date, Trillium has shown that 6/18 patients (33%) saw a response. Importantly there were no serious adverse events. The company is now escalating to 12 mg/kg dose level.
It is difficult to make a head-to-head comparison between Trillium and FourtySeven because (1) Trillium’s data is monotherapy, whereas FourtySeven was in combination with rituximab and (2) the indications are different (TRIL in broad lymphomas, FourtySeven in DLBCL).
The main point of interest in Trillium comes from potential response rates when Trillium’s TTI-622 is combined with existing therapies such as PD-1. Theoretically, if monotherapy TTI-622 shows 25-50% response rates in heavily treated lymphoma patients, combination should be higher. This is, most probably, what drew attention of Pfizer and Wall Street.
Not All CD47 Agents Are Equal
From an efficacy point of view, Trillium’s response rate is promising because it is from a single agent, without added benefits of combination approach (discussed above). Importantly, Trillium has reported a relatively clean safety profile.
Although it is overexpressed on tumor cells, CD47 is also expressed in many normal cells, including red blood cells and platelets. This exposes CD47 antibodies to induce side effects like red blood cell clumping (“hemagglutination”) and anemia, both seen with FourtySeven’s Phase 1 study. Trillium’s candidates, on the other hand, have shown not to bind to red blood cells, which reduces risk of anemia and other blood related adverse events.
The next steps for Trillium are to complete their Phase 1 dose-escalation studies and move on to Phase 2 studies that target specific hematology indications. The good news is that Trillium will have nearly $300 million to advance their pipeline forward.